作者:CY Liu
Monthly case study 202311
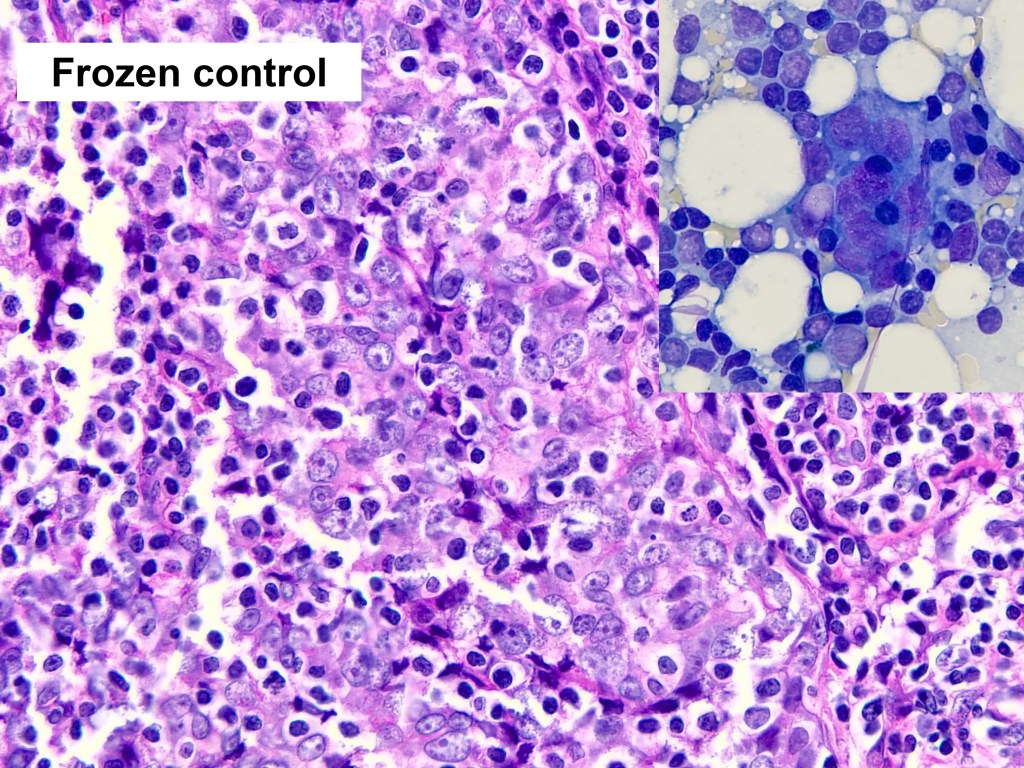
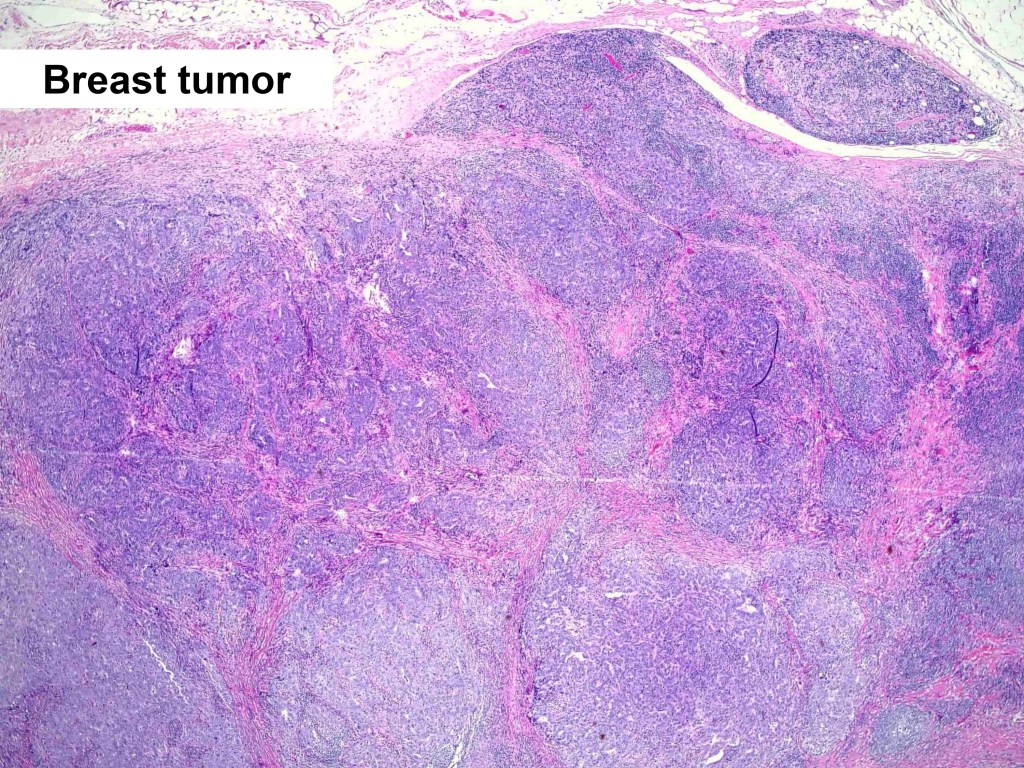
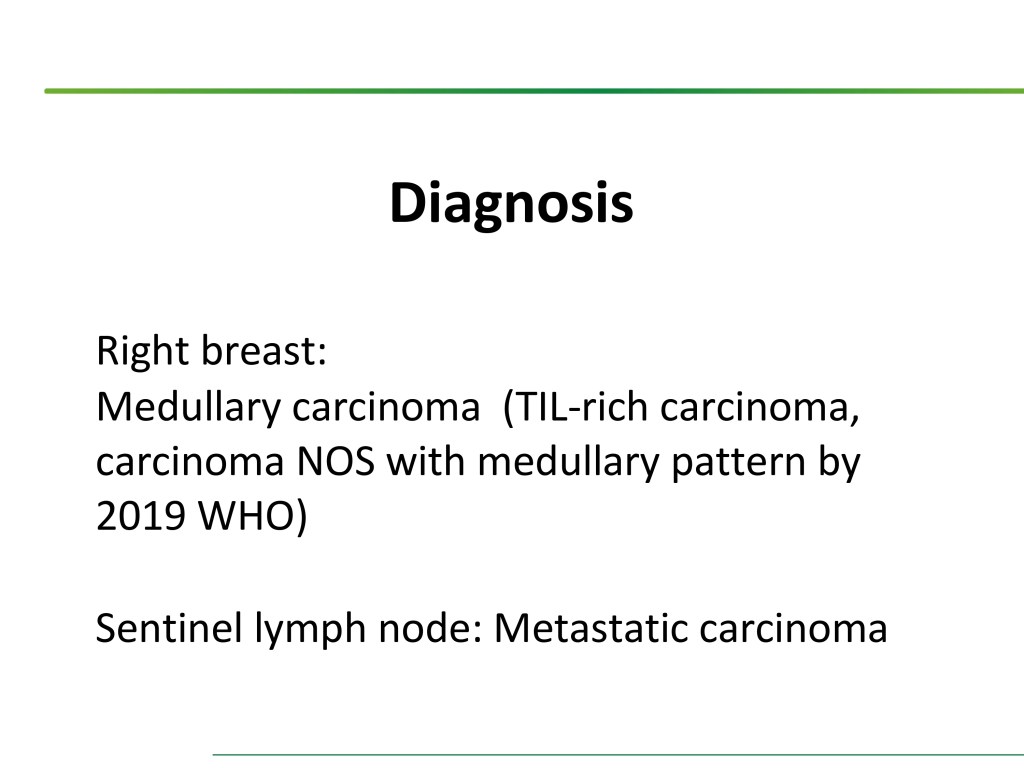
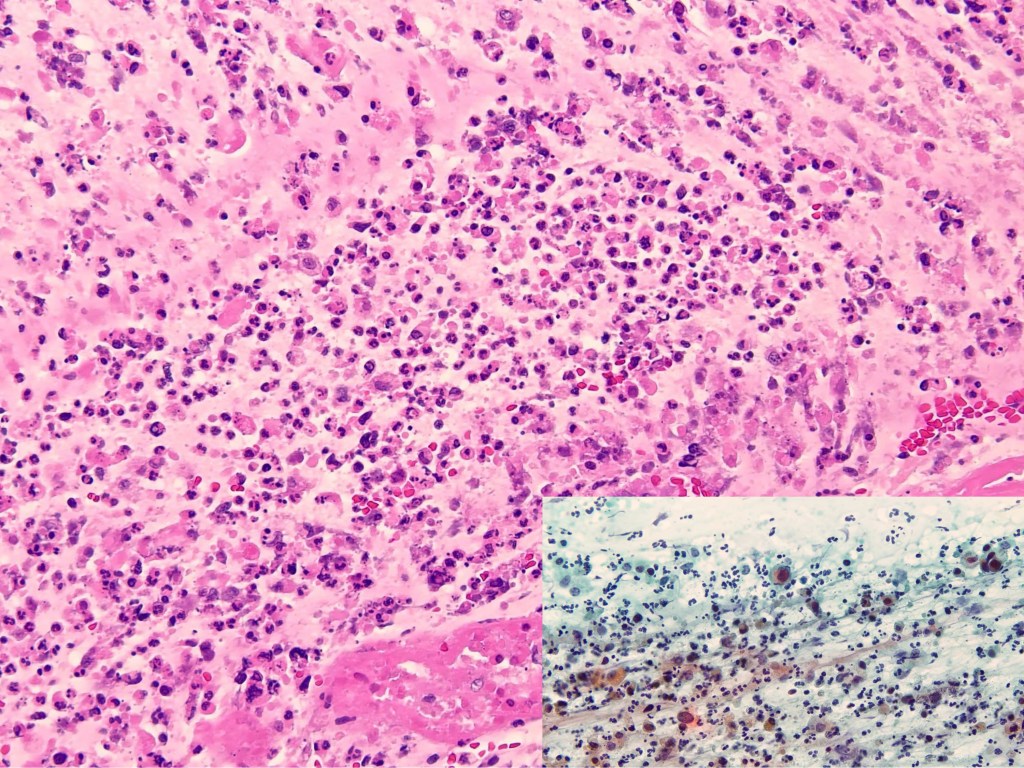
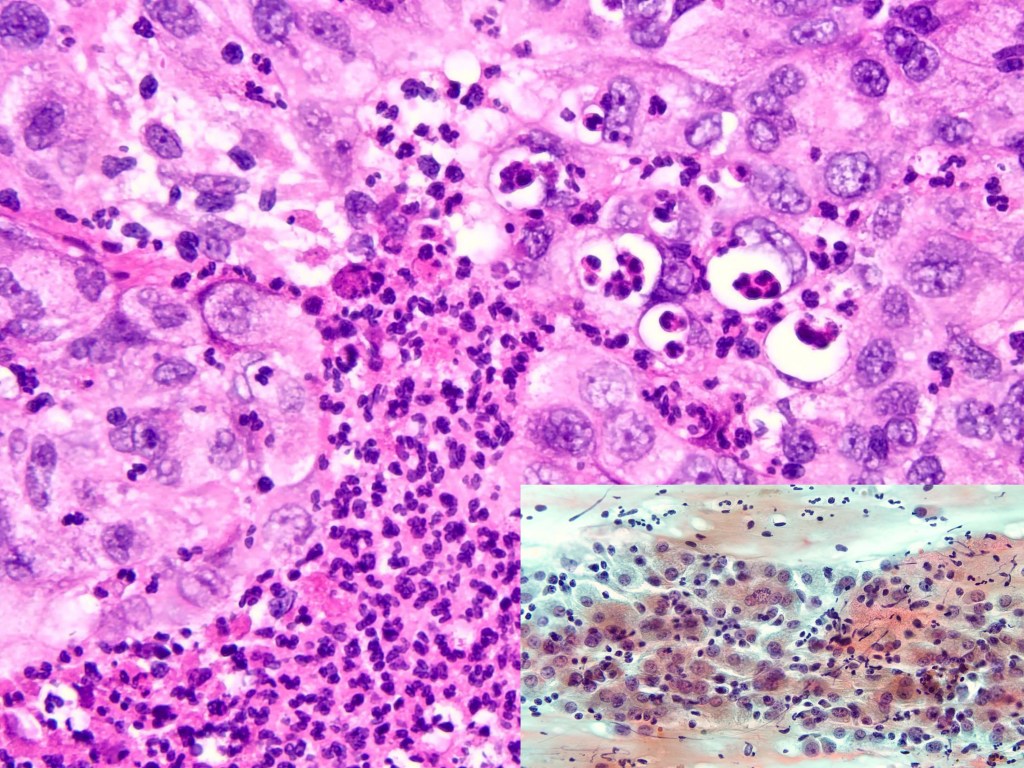
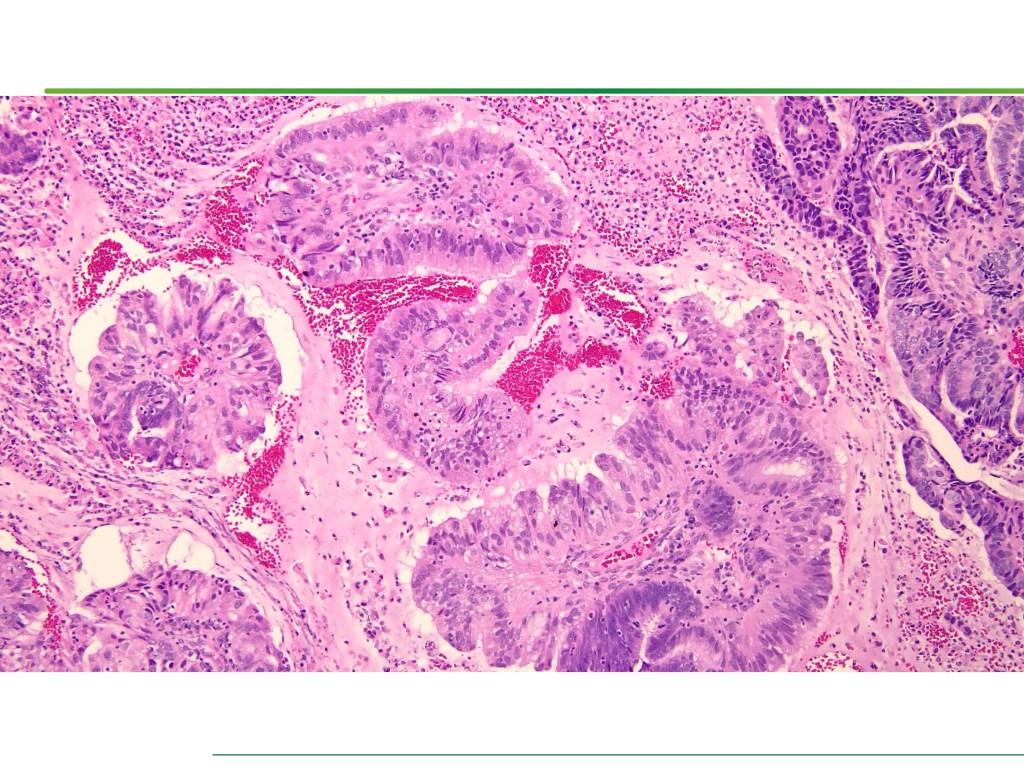
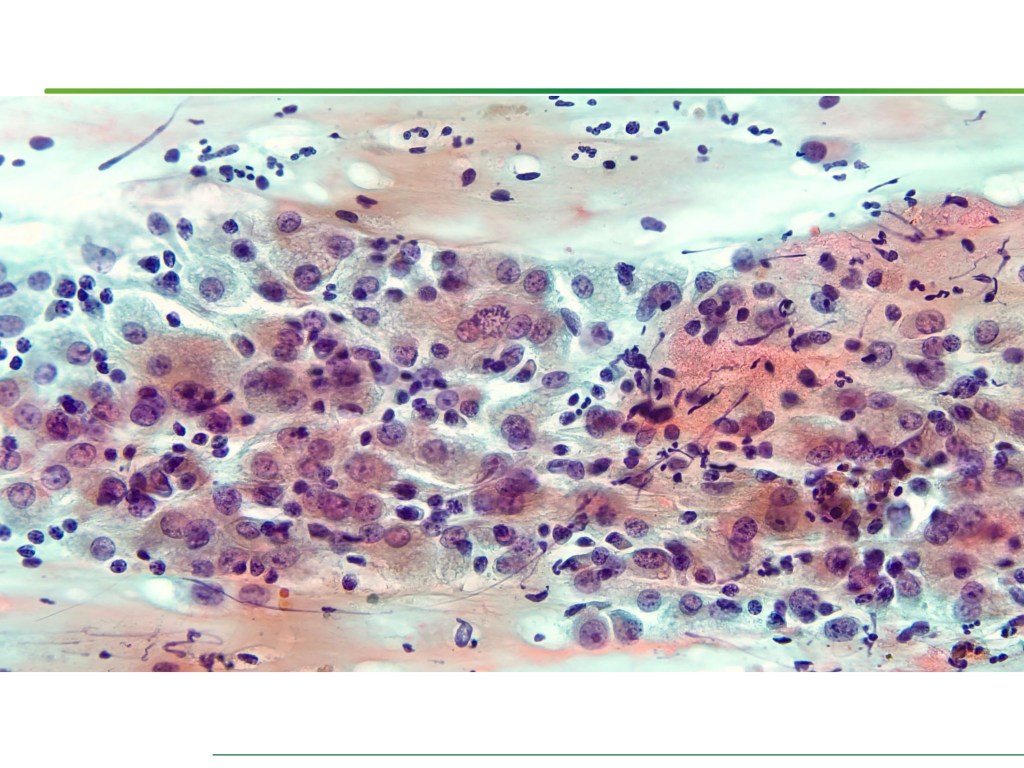
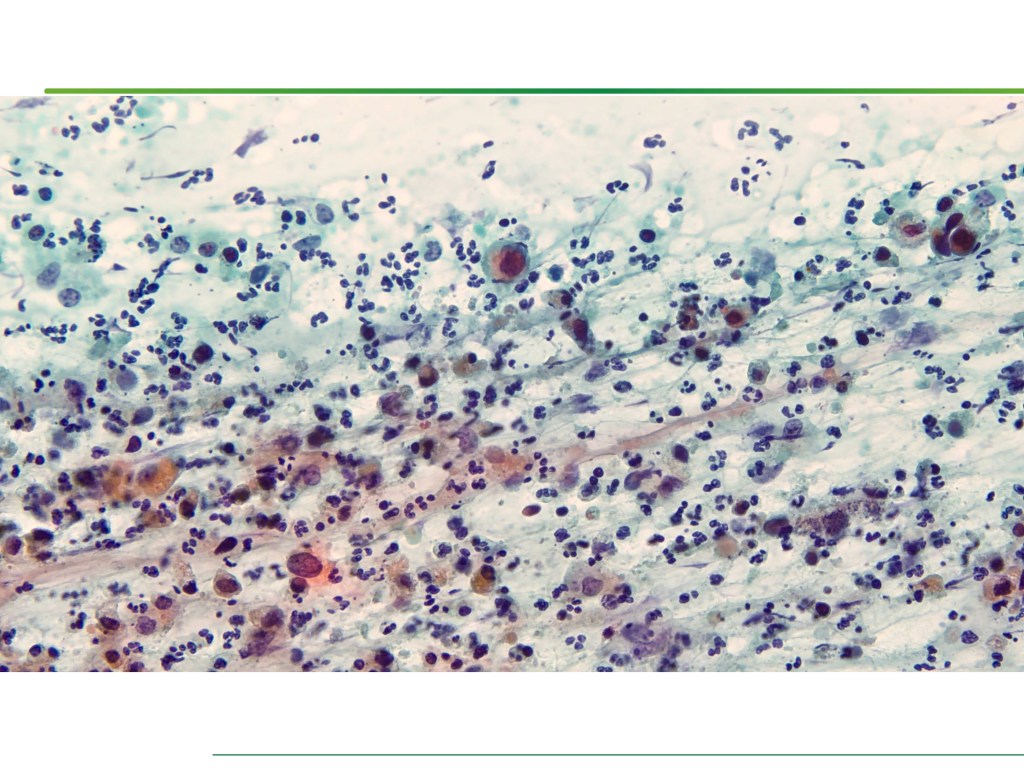
Combined Merkel Cell Carcinoma with Nodal Presentation: Report of a Case Diagnosed with Excisional but Not Incisional Biopsy and Literature Review
Chih-Yi Liu 1 2, Nai-Wen Kang 3, Kengo Takeuchi 4 5 6, Shih-Sung Chuang 7
Affiliations
- Division of Pathology, Sijhih Cathay General Hospital, New Taipei City 221, Taiwan.
- School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City 221, Taiwan.
- Division of Hemato-Oncology, Department of Internal Medicine, Chi-Mei Medical Center, Tainan 710, Taiwan.
- Division of Pathology, Cancer Institute, Japanese Foundation for Cancer Research, Tokyo 135-8550, Japan.
- Pathology Project for Molecular Targets, Cancer Institute, Japanese Foundation for Cancer Research, Tokyo 135-8550, Japan.
- Department of Pathology, Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo 135-8550, Japan.
- Department of Pathology, Chi-Mei Medical Center, Tainan 710, Taiwan.
ABSTRACT
Merkel cell carcinoma (MCC) is a rare primary neuroendocrine carcinoma (NEC) of the skin. As compared to pure MCCs, combined MCCs are aggressive and exhibit a higher probability of metastasis. A correct diagnosis might be missed, especially when the biopsy sample is too small or too superficial. We report a 79-year-old Taiwanese male who presented with lymphadenopathy suspicious for lymphoma. A nodal biopsy showed metastatic NEC. A skin tumor in the lower back was identified, and an incisional biopsy showed only squamous cell carcinoma (SCC). A subsequent excisional biopsy was performed based on the advice of the senior pathologist because of the presence of metastatic nodal NEC. Finally, a diagnosis of combined MCC and SCC was confirmed. Our literature review identified 13 cases of combined MCC with nodal metastasis as initial presentations, all with an aggressive clinical course. Both the MCC and non-MCC components could be present in the metastatic nodes. Metastases of pure MCC cells were observed in three combined MCCs in sun-protected areas, probably pointing to a distinct pathogenesis. Excision or punch biopsy to include the deep dermal NEC component is recommended as timely diagnosis is mandatory for appropriate management of patients with this rare skin cancer.
Keywords: Merkel cell carcinoma; Merkel cell polyomavirus; cytology; paranuclear blue inclusion; skin cancer; thyroid transcription factor-1.
>Diagnostics (Basel). 2023 Jan 26;13(3):449. doi: 10.3390/diagnostics13030449.
Functional Plasmon-Activated Water Increases Akkermansia muciniphila Abundance in Gut Microbiota to Ameliorate Inflammatory Bowel Disease
Chun-Chao Chang 1 2 3, Chih-Yi Liu 4 5, I-Chia Su 1, Yuarn-Jang Lee 1 2, Hsing-Jung Yeh 1 2, Wen-Chao Chen 1, Chih-Jui Yu 1, Wei-Yu Kao 1 2, Yu-Chuan Liu 6 7, Chi-Jung Huang 8 9
Affiliations
- Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei 110, Taiwan.
- Division of Gastroenterology and Hepatology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei 110, Taiwan.
- TMU Research Center for Digestive Medicine, Taipei Medical University, Taipei 110, Taiwan.
- Department of Pathology, Sijhih Cathay General Hospital, New Taipei 221, Taiwan.
- School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei 242, Taiwan.
- Department of Biochemistry and Molecular Cell Biology, School of Medicine, College of Medicine, Taipei Medical University, Taipei 110, Taiwan.
- Cell Physiology and Molecular Image Research Center, Wan Fang Hospital, Taipei Medical University, Taipei 110, Taiwan.
- Department of Medical Research, Cathay General Hospital, Taipei 106, Taiwan.
- Department of Biochemistry, National Defense Medical Center, Taipei 114, Taiwan.
ABSTRACT
Inflammatory bowel disease (IBD) is associated with dysbiosis and intestinal barrier dysfunction, as indicated by epithelial hyperpermeability and high levels of mucosal-associated bacteria. Changes in gut microbiota may be correlated with IBD pathogenesis. Additionally, microbe-based treatments could mitigate clinical IBD symptoms. Plasmon-activated water (PAW) is known to have an anti-inflammatory potential. In this work, we studied the association between the anti-inflammatory ability of PAW and intestinal microbes, thereby improving IBD treatment. We examined the PAW-induced changes in the colonic immune activity and microbiota of mice by immunohistochemistry and next generation sequencing, determined whether drinking PAW can mitigate IBD induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) and dysbiosis through mice animal models. The effects of specific probiotic species on mice with TNBS-induced IBD were also investigated. Experimental results indicated that PAW could change the local inflammation in the intestinal microenvironment. Moreover, the abundance of Akkermansia spp. was degraded in the TNBS-treated mice but elevated in the PAW-drinking mice. Daily rectal injection of Akkermansia muciniphila, a potential probiotic species in Akkermansia spp., also improved the health of the mice. Correspondingly, both PAW consumption and increasing the intestinal abundance of Akkermansia muciniphila can mitigate IBD in mice. These findings indicate that increasing the abundance of Akkermansia muciniphila in the gut through PAW consumption or other methods may mitigate IBD in mice with clinically significant IBD.
Keywords: Akkermansia muciniphila; gut microbiota; inflammatory bowel disease; microbial biomarker; plasmon-activated water.
>Int J Mol Sci. 2022 Sep 28;23(19):11422. doi: 10.3390/ijms231911422.
Intestinal Mucosal Barrier Improvement with Prebiotics: Histological Evaluation of Longish Glucomannan Hydrolysates-Induced Innate T Lymphocyte Activities in Mice
Shih-Chang Chang 1, Hui-Hsun Chiang 2, Chih-Yi Liu 3 4, Yu-Ju Li 5, Chung-Lun Lu 6, Yung-Pin Lee 7, Chi-Jung Huang 8 9, Ching-Long Lai 10 11
Affiliations
- Division of Colorectal Surgery, Department of Surgery, Cathay General Hospital, Taipei 106438, Taiwan.
- School of Nursing, National Defense Medical Center, Taipei 114201, Taiwan.
- Division of Pathology, Sijhih Cathay General Hospital, New Taipei City 221037, Taiwan.
- School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City 242062, Taiwan.
- Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung 80756, Taiwan.
- Aquatic Technology Research Center, Agricultural Technology Research Institute, Xiangshan, sinchu 300110, Taiwan.
- Research and Development, Healthy-Bioceuticals Company, Taipei 114201, Taiwan.
- Department of Medical Research, Cathay General Hospital, Taipei 106438, Taiwan.
- Department of Biochemistry, National Defense Medical Center, Taipei 114201, Taiwan.
- Division of Basic Medical Sciences, Department of Nursing, Chang Gung University of Science and Technology, Taoyuan 333324, Taiwan.
- Research Center for Chinese Herbal Medicine, Chang Gung University of Science and Technology, Taoyuan 333324, Taiwan.
ABSTRACT
Use of prebiotics is a growing topic in healthcare. A lightweight molecule and water-soluble fiber ingredient, longish glucomannan hydrolysates (LGH), has been developed to improve the intestinal mucosal barrier and confer gut health benefits. This study aims to investigate the implications of continuous LGH intervening in intestinal epithelium integrity and protective immunity against chemical dextran sodium sulfate (DSS)-induced colitis. Twelve male BALB/c mice were randomly arranged into four groups. The LGH/DSS group had results in bodyweight variance, epithelial cell density, and aberrancy score as good as the LGH group, and both were equivalent to the control group. LGH consumption effectively protects the distal intestinal epithelium by activating innate T lymphocytes. Meanwhile, T-cell subsets in subepithelial interspersion take a bystander role in these microenvironmental alterations. Under this stress, the cluster of differentiation 3 (CD3)+ T cells infiltrate the epithelium, while CD4+ T cells inversely appear in submucosal large lymphoid aggregates/isolated lymphoid follicles (ILFs) in which significant CD3+, CD4+, and CD8+ T-cell populations agglomerate. Moreover, forkhead box P3 (Foxp3) and interleukin 17 (IL-17) are observed in these ILFs. Agglomerated CD4+ T-cell lineages may have roles with proinflammatory T helper 17 cells and anti-inflammatory regulatory T cells in balancing responses to intraluminal antigens. Collectively, LGH administration may function in immune modulation to protect against DSS-induced inflammation.
Keywords: T-cell activation; colitis; glucomannan; lymphoid aggregates; prebiotics.
>Nutrients. 2022 May 26;14(11):2220. doi: 10.3390/nu14112220.
Lithosepermic Acid Restored the Skin Barrier Functions in the Imiquimod-Induced Psoriasis-like Animal Model
Li-Ching Chen 1, Yu-Ping Cheng 2, Chih-Yi Liu 3, Jiun-Wen Guo 4
Affiliations
- Division of Infectious Diseases, Cathay General Hospital, Taipei 10630, Taiwan.
- Department of Dermatology, Cathay General Hospital, Taipei 10630, Taiwan.
- Division of Pathology, Sijhih Cathay General Hospital, New Taipei City 22174, Taiwan.
- Department of Medical Research, Cathay General Hospital, Taipei 10630, Taiwan.
ABSTRACT
(1) Background: Psoriasis is a T helper 1/T helper 17 cells-involved immune-mediated genetic disease. Lithospermic acid, one of the major phenolic acid compounds of Danshen, has antioxidation and anti-inflammation abilities. Due to the inappropriate molecular weight for topical penetration through the stratum corneum, lithospermic acid was loaded into the well-developed microemulsion delivery system for IMQ-induced psoriasis-like dermatitis treatment. (2) Methods: BALB/c mice were administered with topical imiquimod to induce psoriasis-like dermatitis. Skin barrier function, disease severity, histology assessment, autophagy-related protein expression, and skin and spleen cytokine expression were evaluated. (3) Results: The morphology, histopathology, and skin barrier function results showed that 0.1% lithospermic acid treatment ameliorated the IMQ-induced psoriasis-like dermatitis and restored the skin barrier function. The cytokines array results confirmed that 0.1% lithospermic acid treatment inhibited the cutaneous T helper-17/Interleukin-23 axis related cytokines cascades. (4) Conclusions: The results implied that lithospermic acid might represent a possible new therapeutic agent for psoriasis treatment.
Keywords: autophagy; inflammatory cytokines; lithospermic acid; psoriasis; skin barrier.
>Int J Mol Sci. 2022 May 31;23(11):6172. doi: 10.3390/ijms23116172.
Butyrate supplementation regulates expression of chromosome segregation 1‑like protein to reverse the genetic distortion caused by p53 mutations in colorectal cancer
Chun-Chao Chang 1, Wei-Yu Kao 1, Chih-Yi Liu 2, Hui-Hsien Su 1, Yu-An Kan 1, Pao-Ying Lin 1, Wei-Chi Ku 3, Kang-Wei Chang 4, Ruey-Neng Yang 3, Chi-Jung Huang 5
Affiliations
- Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei 11031, Taiwan, R.O.C.
- Department of Pathology, Sijhih Cathay General Hospital, New Taipei 22174, Taiwan, R.O.C.
- School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei 24205, Taiwan, R.O.C.
- Neuroscience Research Center, Taipei Medical University, Taipei 11031, Taiwan, R.O.C.
- Department of Medical Research, Cathay General Hospital, Taipei 10630, Taiwan, R.O.C.
ABSTRACT
The chromosome segregation 1‑like (CSE1L) protein, which regulates cellular mitosis and apoptosis, was previously found to be overexpressed in colorectal cancer (CRC) cells harboring mutations. Therefore, regulating CSE1L expression may confer chemotherapeutic effects against CRC. The gut microflora can regulate gene expression in colonic cells. In particular, metabolites produced by the gut microflora, including the short‑chain fatty acid butyrate, have been shown to reduce CRC risk. Butyrates may exert antioncogenic potential in CRC cells by modulating p53 expression. The present study evaluated the association between CSE1L expression and butyrate treatment from two non‑transformed colon cell lines (CCD‑18Co and FHC) and six CRC cell lines (LS 174T, HCT116 p53+/+, HCT116 p53‑/‑, Caco‑2, SW480 and SW620). Lentiviral knockdown of CSE1L and p53, reverse transcription‑quantitative PCR (CSE1L, c‑Myc and p53), western blotting [CSE1L, p53, cyclin (CCN) A2, CCNB2 and CCND1], wound healing assay (cell migration), flow cytometry (cell cycle analysis) and immunofluorescence staining (CSE1L and tubulin) were adopted to verify the effects of butyrate on CSE1L‑expressing CRC cells. The butyrate‑producing gut bacteria Butyricicoccus pullicaecorum was administered to mice with 1,2‑dimethylhydrazine‑induced colon tumors before the measurement of CSE1L expression. The effects of B. pullicaecorum on CSE1L expression were then assessed by immunohistochemical staining for CSE1L and p53 in tissues from CRC‑bearing mice. Non‑cancerous colon cells with the R273H p53 mutation or CRC cells haboring p53 mutations were found to exhibit significantly higher CSE1L expression levels. CSE1L knockdown in HCT116 p53‑/‑ cells resulted in G1‑and G2/M‑phase cell cycle arrest. Furthermore, in HCT116 p53‑/‑ cells, CSE1L expression was already high at interphase, increased at prophase, peaked during metaphase before declining at cytokinesis but remained relatively high compared with that in HCT116 expressing wild‑type p53. Significantly decreased expression levels of CSE1L were also observed in HCT116 p53‑/‑ cells that were treated with butyrate for 24 h. In addition, the migration of HCT116 p53‑/‑ cells was significantly decreased after CSE1L knockdown or butyrate treatment. Tumors with more intense nuclear p53 staining and weaker CSE1L staining were found in mice bearing DMH/DSS‑induced CRC that were administered with B. pullicaecorum. Taken together, the results indicated that butyrate can impair CSE1L‑induced tumorigenic potential. In conclusion, butyrate‑producing microbes, such as B. pullicaecorum, may reverse the genetic distortion caused by p53 mutations in CRC by regulating CSE1L expression levels.
Keywords: butyricicoccus pullicaecorum; chromosome segregation 1‑like; colorectal cancer; p53 tumor suppressor; short‑chain fatty acid.
>Int J Oncol. 2022 Jun;60(6):64. doi: 10.3892/ijo.2022.5354. Epub 2022 Apr 13.
Primary Effusion Lymphoma: A Timely Review on the Association with HIV, HHV8, and EBV
Chih-Yi Liu 1 2, Bo-Jung Chen 3 4, Shih-Sung Chuang 5
Affiliations
- Division of Pathology, Sijhih Cathay General Hospital, New Taipei City 221, Taiwan.
- School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City 221, Taiwan.
- Department of Pathology, Shuang Ho Hospital, Taipei Medical University, New Taipei City 221, Taiwan.
- Department of Pathology, School of Medicine, College of Medicine, Taipei Medical University, Taipei 110, Taiwan.
- Department of Pathology, Chi-Mei Medical Center, Tainan 710, Taiwan.
ABSTRACT
Primary effusion lymphoma (PEL) is defined by the WHO classification as a large B-cell neoplasm without detectable tumor masses. It is universally associated with HHV8, with most cases occurring in the setting of immunodeficiency such as HIV infection, and a poor prognosis. Morphologically, the neoplastic cells range from immunoblastic, plasmablastic, to anaplastic; and phenotypically, most cases express plasma cell but not B-cell markers, i.e., plasmablastic. During the past decade, primary HHV8-negative effusion lymphoma has been reported. Such cases were considered in the WHO classification scheme as effusion-based lymphoma. We performed a systemic review of 167 HHV8-negative effusion lymphomas from the literature and found that only 42% were associated with a fluid overload state, and with low rates of HIV (6%) or EBV (21%) infection. Furthermore, most patients are old (or immunosenescent) with underlying medical conditions/comorbidities, most neoplasms are of B-cell phenotype, and the outcome is more favorable than that of HHV8-positive PEL. These distinctive findings supported our prior proposal of designating these HHV8-negative cases as type II PEL, in contrast to the classic or type I PEL as defined by the WHO. Furthermore, we propose an algorithmic approach for the diagnosis of PEL and its mimickers.
Keywords: EBV; HHV8; HIV; effusion-based lymphoma; primary effusion lymphoma.
< Review >Diagnostics (Basel). 2022 Mar 15;12(3):713. doi: 10.3390/diagnostics12030713.
Deciphering Genetic Alterations of Taiwanese Patients with Pancreatic Adenocarcinoma through Targeted Sequencing
Chi-Cheng Huang 1 2 3, Chih-Yi Liu 4, Chi-Jung Huang 5 6, Yao-Chun Hsu 7, Heng-Hui Lien 8 9, Jia-Uei Wong 10, Feng-Chuan Tai 8, Wen-Hui Ku 11, Chi-Feng Hung 9, Jaw-Town Lin 12, Ching-Shui Huang 8 13, Han-Sun Chiang 9
Affiliations
- Division of General Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei 11217, Taiwan.
- Comprehensive Breast Health Center, Taipei Veterans General Hospital, Taipei 11217, Taiwan.
- Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei 100, Taiwan.
- Department of Pathology, Cathay General Hospital SiJhih, New Taipei 221, Taiwan.
- Department of Medical Research, Cathay General Hospital, Taipei 106, Taiwan.
- Department of Biochemistry, National Defense Medical Center, Taipei 114, Taiwan.
- Division of Gastroenterology, Department of Internal Medicine, E-da Hospital, Kaohsiung 82445, Taiwan.
- Division of General Surgery, Department of Surgery, Cathay General Hospital, Taipei 106, Taiwan.
- School of Medicine, College of Medicine, Fu-Jen Catholic University, New Taipei 242, Taiwan.
- Division of General Surgery, Department of Surgery, Fu-Jen Catholic University Hospital, New Taipei 243, Taiwan.
- Department of Clinical Pathology and Molecular Medicine, Taipei Institute of Pathology, Taipei 10374, Taiwan.
- Digestive Medicine Center, China Medical University Hospital, Taichung 404, Taiwan.
- School of Medicine, College of Medicine, Taipei Medical University, Taipei 110, Taiwan.
ABSTRACT
Pancreatic adenocarcinoma (PAC) is the 8th leading cause of cancer-related deaths in Taiwan, and its incidence is increasing. The development of PAC involves successive accumulation of multiple genetic alterations. Understanding the molecular pathogenesis and heterogeneity of PAC may facilitate personalized treatment for PAC and identify therapeutic agents. We performed tumor-only next-generation sequencing (NGS) with targeted panels to explore the molecular changes underlying PAC patients in Taiwan. The Ion Torrent Oncomine Comprehensive Panel (OCP) was used for PAC metastatic lesions, and more PAC samples were sequenced with the Ion AmpliSeq Cancer Hot Spot (CHP) v2 panel. Five formalin-fixed paraffin-embedded (FFPE) metastatic PAC specimens were successfully assayed with OCP, and KRAS was the most prevalent alteration, which might contraindicate the use of anti-EGFR therapy. One PAC patient harbored a FGFR2 p. C382R mutation, which might benefit from FGFR tyrosine kinase inhibitors. An additional 38 samples assayed with CHP v2 showed 100 hotspot variants, collapsing to 54 COSMID IDs. The most frequently mutated genes were TP53, KRAS, and PDGFRA (29, 23, 10 hotspot variants), impacting 11, 23, and 10 PAC patients. Highly pathogenic variants, including COSM22413 (PDGFRA, FATHMM predicted score: 0.88), COSM520, COSM521, and COSM518 (KRAS, FATHMM predicted score: 0.98), were reported. By using NGS with targeted panels, somatic mutations with therapeutic potential were identified. The combination of clinical and genetic information is useful for decision making and precise selection of targeted medicine.
Keywords: Taiwan; actionable mutation; next-generation sequencing; pancreatic adenocarcinoma; targeted sequencing.
>Int J Mol Sci. 2022 Jan 29;23(3):1579. doi: 10.3390/ijms23031579.
The Periodontopathic Pathogen, Porphyromonas gingivalis, Involves a Gut Inflammatory Response and Exacerbates Inflammatory Bowel Disease
Yu-Chen Lee 1, Chih-Yi Liu 2 3, Chia-Long Lee 4, Ruo-Han Zhang 5 6, Chi-Jung Huang 7 8, Ting-Lin Yen 6 7 9
Affiliations
- Department of Dentistry, Cathay General Hospital, Taipei 106, Taiwan.
- Division of Pathology, Sijhih Cathay General Hospital, New Taipei City 221, Taiwan.
- School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City 242, Taiwan.
- Department of Internal Medicine, Cathay General Hospital, Taipei 106, Taiwan.
- Graduate School of Health Industry Management, Ching Kuo Institute of Management and Health, Keelung City 20301, Taiwan.
- Department of Oral Hygiene Care, Ching Kuo Institute of Management and Health, Keelung City 20301, Taiwan.
- Department of Medical Research, Cathay General Hospital, Taipei 106, Taiwan.
- Department of Biochemistry, National Defense Medical Center, Taipei 114, Taiwan.
- Department of Pharmacology, School of Medicine, College of Medicine, Taipei Medical University, Taipei 110, Taiwan.
ABSTRACT
Periodontal disease (PD) is one of the most prevalent disorders globally and is strongly associated with many other diseases. Inflammatory bowel disease (IBD), an inflammatory condition of the colon and the small intestine, is reported to be associated with PD through undetermined mechanisms. We analyzed taxonomic assignment files from the Crohn’s Disease Viral and Microbial Metagenome Project (PRJEB3206). The abundance of Porphyromonadaceae in fecal samples was significantly different between patients with Crohn’s disease and control volunteers. Dextran sulfate sodium was used to induce colitis in mice to reveal the effect of this periodontopathic pathogen in vivo. After intrarectal implantation of Porphyromonas gingivalis (Pg)-the primary pathogen causing PD-the disease activity index score, colonic epithelial loss, and inflammatory cell infiltration were intensified. In addition, tumor necrosis factor-α and interleukin-6 showed the highest levels in Pg-infected colons. This revealed the importance of Pg in the exacerbation of IBD. Thus, simultaneous treatment of PD should be considered for people with IBD. Moreover, implantation of Pg in the rectum worsened the clinical symptoms of colitis in mice. Because Pg participates in the pathogenesis of IBD, reducing the chances of it entering the intestine might prevent the worsening of this disorder.
Keywords: Porphyromonas gingivalis; gut inflammation; inflammatory bowel disease; periodontal disease.
>Pathogens. 2022 Jan 11;11(1):84. doi: 10.3390/pathogens11010084.